Are Fuel Cells Commercially Viable?
Fuel cells were invented in the 19th century, but their practical application has been extremely limited to date, as thermal power generation has become the mainstream method of power generation. Today, with decarbonization and the hydrogen economy, fuel cells are again attracting attention as a method of power generation using hydrogen. Innovations are necessary for fuel cells to become more practical than they are today, not only in the fuel cells themselves but also in the way of storing and transporting their fuel, as well as in the method of producing it.

1. Preface
Before telling whether a hydrogen-powered fuel cell engine is superior to an oil-powered internal combustion engine as a generator, let me explain what a fuel cell is.
A fuel cell is an electrochemical cell in which the energy of a reaction between a fuel (typically hydrogen) and an oxidant (typically oxygen) is converted directly and continuously into electrical energy.

On the anode side, hydrogen diffuses to the anode catalyst where it dissociates into protons (H+) and electrons (e–). The protons are conducted through the electrolyte membrane to the cathode, but the electrons are forced to travel in an external circuit, supplying power, because the membrane is electrically insulating. On the cathode catalyst, oxygen molecules react with the electrons and protons to form water.
You might think that burning hydrogen directly is an easier way to utilize it as fuel, but such a high-temperature reaction oxidizes nitrogen, the main constituent of the atmosphere, and produces harmful nitrogen oxides.
There are many types of fuel cells but Polymer Electrolyte Fuel Cells (PEFC) are the most promising among them. They operate at relatively low temperatures, around 80 (25-100) °C. Owing to their low-temperature operation they can start quickly and system components are less worn out. They are developed for automotive use, so the market size for that use is enormous.
2. The Merits of Fuel Cells
The reason why fuel cells are regarded as the best is as follows.
2.1. Highly Efficient
Theoretically, fuel cells can have higher efficiencies in generating electrical energy than thermal power plants, because they do not operate with a thermal cycle. The maximum theoretical efficiencies for fuel cells can be more than 80%, but the real-world efficiencies are about half of the former, because of polarization inside them.
To increase practical efficiency, cogeneration is necessary. Cogeneration is the use of a power station to simultaneously generate both heat and electricity. You can use the generated heat not only for heating but also for cooling. You don’t have to consume energy to run air conditioners or boil water for its own sake.
The fuel cells are the distributed power generators, small, clean, and safe enough to be placed in a location where heat is also needed. The conventional centralized power generators, typically nuclear power plants, are too large, dirty, or dangerous to be installed in our houses or offices.
Cogeneration is most efficient when the heat can be used near the place where it is generated, because transporting heat over long distances is expensive, unlike electricity. Because the centralized power generators are located far from energy consumers, they can hardly utilize most of the produced heat, so their energy efficiency remains low. Although some heat is lost with the exhaust gas also in fuel cells, their fuel-to-electricity conversion efficiency is 40% and their fuel-to-heat conversion efficiency is 40% so that their combined efficiency amounts to about 80%.
Cogeneration by fuel cells has already been put into practical use. Fujitsu inaugurated a 200-kilowatt hydrogen fuel cell at its Sunnyvale campus in California in 2007. Fujitsu uses the heat harvested from the reactions required to turn hydrogen into electricity so that the efficiency of the fuel cell can be raised to 85 percent.[2]
The demerit of cogeneration is that the heat demand seldom matches the electricity demand. Cogeneration can be designed for the demand for either heat or electricity. The heat-driven operation seems to be better, for it is more difficult to transport heat than electricity. You can use fuel cells at home to produce heat and sell excess electric power back to the grid if permitted by the network owner.
If every home, office, and factory can sell excess electric power back to the grid, this interactive network of selling and buying electricity promotes competition and the perfect competition would lead to a completely efficient outcome. Electricity has long been regarded as an example of natural monopolies, where capital costs predominate, creating economies of scale and hence high barriers to entry. The interactive network can break this monopoly.
On the Internet, each independent host can choose which Internet services to use and which local services to make available to the global Internet community. If you program your computer on the Internet to automatically select the cheapest electricity seller, the competition will reduce the price of electricity.
The rival of cogeneration is heat pumps. A heat pump is a machine or device that moves heat from one location to another via work, that is to say, by evaporating and condensing fluid known as a refrigerant. It is a reverse Carnot cycle. While the Carnot cycle transforms high-temperature heat into electricity and waste heat, the heat pump transforms electricity and waste heat into high-temperature heat. As I have already explained the Carnot cycle, I will explain the reverse Carnot cycle in a similar way.
The thermal energy of a gas system is called internal energy. The internal energy of refrigerant U increases when heat Q is added to it or work W is done on it.
ΔU=W+Q
Heat pumps follow the four stages of the Carnot cycle reversely:
- Adiabatic Compression: A compressor pressurizes the refrigerant. As the work is done on the refrigerant, its internal energy increases.
- Isothermal Compression: The hot and highly pressurized gas is cooled in a heat exchanger. As the heat is reduced, its internal energy decreases.
- Adiabatic Expansion: The condensed refrigerant passes through a pressure-lowering device and expands. As the refrigerant does the work, its internal energy decreases.
- Isothermal Expansion: The cold refrigerant flows to another heat exchanger, absorbs heat, and evaporates. As the heat is added, its internal energy increases.
The refrigerant then returns to the compressor and the cycle is repeated. A heat pump is energy saving because it makes use of the heat of the atmosphere at the isothermal expansion stage. The coefficient of performance, the ratio of the output heat to the supplied work, is nowadays over 3.
Heat pumps have, however, some problems. As the atmospheric heat is thinly scattered, it takes a long time to collect enough heat. It cannot generate much heat at once. You can reserve a lot of hot water if you enlarge the heat pump device, but it costs a lot and requires a lot of space.
The running cost of a heat pump is low, but the front-end cost is high. The life of it is uncertain because it is a high-pressure device and cannot be free from trouble.
The coefficient of performance drops, where it is cold and heat is difficult to collect. It might break down under minus 20°C. The colder it is, the more heat is demanded. So, we cannot depend on heat pumps in the very cold district.
The problem of cogeneration designed for the demand of heat is that it makes fuel cells intermittent power generators. Renewable energy, such as wind power, geothermal power, solar power, tidal power, wave power, and so on are all intermittent. If we introduce intermittent electricity into the grid, we cannot fill the gap between demand and supply. So, we need fuel cells that generate only electricity. As I will explain, we can utilize heat from some fuel cells to produce hydrogen by thermolysis of water.
2.2. Clean and Silent
Burning oil or coal releases noxious gases, such as nitrogen oxides, sulfur dioxide, carbon monoxide, and chemical vapors. Natural gas is cleaner than oil and coal but still its burning releases nitrogen oxides. These exhaust gases are not only directly harmful to human health but also result in acid deposition and photochemical smog as they take part in further chemical reactions in the atmosphere.
The acid deposition occurs when nitrogen oxides (NOx) and sulfur dioxide (SO2) react in the atmosphere with water, oxygen, and other chemicals to form various acidic compounds, for example, like this:
2NO + O2 → 2NO2
2NO2 + H2O → HNO2 + HNO3
SO2 + H2O → H2SO3
2H2SO3 + O2 → 2H2SO4
Acid deposition causes acidification of water and damage to trees, buildings, and paints
Photochemical smog is the chemical reaction of sunlight, nitric oxides, and volatile organic compounds (vapors released from gasoline, paints, solvents, pesticides, and other chemicals) in the atmosphere, which leaves airborne particulate matter and ground-level ozone.
Fuel Cells are clean engines because they emit no nitrogen oxides, no sulfur dioxide, no carbon monoxide, and no chemical vapors except water. They do not emit carbon dioxide, a cause of global warming, either. Water vapor fuel cells emit is a greenhouse gas, but it does not worsen the problem, because saturated water vapor returns to the ground as rain.
Fuel cells are not only clean but also silent. Those who live close to the busy highways complain about road noise. They have constructed barrier walls along highways to keep noise levels down and improve their quality of life. If all cars install the fuel cell engine, such expensive protectors would be no more necessary. Fuel cells have no major moving parts. They convert chemical energy into electrical energy directly and efficiently without making hardly any noise and throb.
2.3. Safe and Stable
Fuel cells are more stable than thermoelectric power plants. Because fuel cells do not use combustion and have no moving parts, their reliability is high and has hardly any downtime in operation. According to Fuel Cells 2000, “Properly configured fuel cells can achieve up to 99.9999% reliability, less than one minute of downtime in a six-year period.[3]“
They are even more stable than batteries. The electrodes of batteries chemically react and change, while those of fuel cells just function as catalysis, when they are charged or discharged.
Of course, fuel cells also can break down. But, as they are the distributed power generators, they are far safer than the current centralized power generators. The more distributed the generators are, the less risky they are. If a centralized power generator breaks down, a blackout will paralyze consumers in the wide area, but this is not the case with distributed power generators.
In this respect, the fuel cell grid is like the Internet, which originally ARPANET developed for military purposes. An attack on any host computer on the Internet cannot paralyze the whole network. Any host computer on the Internet can send and receive information.
Fuel cell systems decentralized power generator, while the Internet decentralized media, as Jeremy Rifkin says:
When millions of small power plants are concerned into vast energy webs using the same architectural design principles and smart technologies that made possible the World Wide Web, people can share energy and sell it to one another ― peer-to-peer energy sharing ― and break the hold of giant energy and power companies forever.[4]
The fuel cell grid shares this toughness and interactivity with the Internet. Every energy consumer can be a producer and if your generator breaks down, you can buy energy from a neighbor on the grid.
Generally speaking, cooling results in centralization of power, while warming decentralization. The Little Ice Age in modern times brought about absolute monarchism and the Industrial Revolution, where the centralized power generator prevails. Today’s global warming brought about democracy and the Information Revolution, where the decentralized power generator prevails.
3. The Problems to Be Solved
I have explained the advantages of fuel cells. But, of course, there are many problems that prevent fuel cells from commercialization. I will classify the problems under four topics and propose solutions to them.
3.1. How to Produce Hydrogen
Hydrogen does not exist in nature as such. If producing hydrogen costs a lot or destroys the environment, it would be nonsense to use fuel cells. The energy sources must be renewable as well to realize sustainable civilization.
The current most economical and widely adopted method is the steam reforming of natural gas. At high temperatures, steam (water vapor) reacts with natural gas (methane) to yield hydrogen and carbon dioxide.
CH4+2H2O → 4H2+CO2
Those who are concerned with global warming do not support this method, because it emits carbon dioxide.
Moreover, natural gas is an exhaustible fossil fuel. There are, however, inexhaustible hydrogen resources that you can find everywhere and get for free; that is the organic waste, such as kitchen garbage, excrement or dead bodies of animals, sludge, agriculture and forestry residue, petrochemical products (plastics, artificial fibers, etc) and so on.
For example, you can ferment the organic waste and get methane as biogas. The organic wastes, when put into the sealed container, generate various gases such as methane, carbon dioxide, ammonia, etc. of which methane occupies 50-60%, as a result of the anaerobic fermentation of bacteria.
Masaru Ichikawa, professor of Hokkaido University, invented catalytic technology of converting biogas-derived methane to hydrogen and benzene without emitting carbon dioxide.[5]
6CH4→C6H6+9H2
The technology is called MTB (Methane to Benzene). The zeolite-supported molybdenum and rhenium catalyze this reaction. Benzene is an important industrial solvent and precursor in the production of drugs, plastics, gasoline, synthetic rubber, dyes, and so on.
From 2003 through 2005, Civil Engineering Research Institute for Cold Region built a demo-pilot plant at Bekkai, Hokkaido, Japan, and succeeded in producing hydrogen and benzene from biogas-derived methane. They fermented cow dung to get methane. As its residue still contains phosphoric acid and other nutrients, it can serve as manure.
This MTB technology can produce hydrogen and benzene (and other petrochemical products) much more inexpensively than the current technology; the cost of hydrogen is $0.1 per cubic meter and that of benzene is $0.3 per kilogram. It is not only ecological but also economical.
The MTB technology has much to be improved. The conversion rate is only 15% per process. They repeated the process several times, converting 45%, then converted the remaining methane to hydrogen and carbon dioxide by steam reforming. As the greenhouse effect of methane is 20 times as strong as that of carbon dioxide, it still contributes to a reduction of the greenhouse effect, but it is desirable to improve the conversion rate.
In 2000, the National Institute of Advanced Industrial Science and Technology (AIST) in Japan developed a new catalyst and raised the rate up to 55% and that of converting only to hydrogen up to 90%. More endeavors to raise the rate are necessary.
The methane fermentation has also much to be improved. The usual method can utilize only limited kinds of materials, takes 25 days to ferment the materials, ferment only 60%, leaves much residue and its recovery rate of energy is low (40-46%).
In 2004, AIST developed a two-stage fermentation that makes anaerobic microbes first produce hydrogen and then methane. They can even ferment paper. This new method takes only 15 days to complete the entire process, ferment 80%, and thus raises the recovery rate of energy up to 55%.
The fermentation method is useful in rural areas whose demand for manure is great, but not suitable for urban areas. The microbes cannot ferment petrochemical wastes the urban areas put out so much. To dispose of organic wastes including petrochemicals, thermal gasification is the most recommended.
Unlike biological processes, gasification operates at elevated temperatures. The temperature is so high that the pollutants contained within the feed waste material are completely cracked into harmless compounds. The mineral fraction is reduced to vitrified glass that can be used for road construction. The organic material can be converted to hydrogen and carbon monoxide that can be used for fuel cells.
The gasification plant can be small enough to be located within limited spaces such as basements in buildings. Organic waste is produced where energy is demanded. This means it costs very little to transport the material of hydrogen to the consumers.
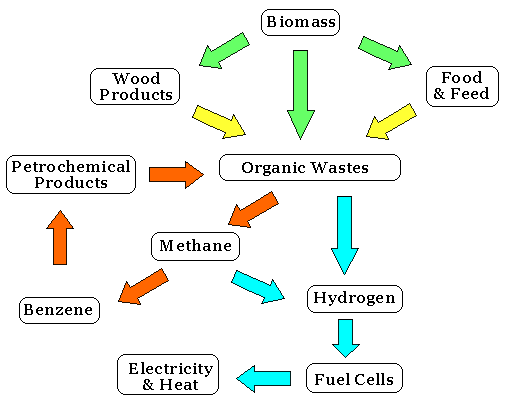
Most of the proponents of the hydrogen economy dream of making solar power or wind power, both of which cannot provide constant electricity, electrolyze water into hydrogen and oxygen, a completely carbon-free hydrogen generation.
But the electrolysis of water is too expensive and too inefficient to commercialize. An electrolyzer and fuel cell would return less than one-third of the input energy, while a much cheaper lead-acid battery can return about 90 percent.
Moreover, solar or wind power generation itself is expensive and inefficient. A completely carbon-free hydrogen economy is just a dream. We should make use of biomass because plants can more efficiently transform solar energy into chemical energy than we can.
Thermolysis is more efficient than electrolysis to make hydrogen from water. The most famous way to decompose water by heat is the Sulfur-Iodine cycle. The S-I cycle consists of the following three chemical reactions.
I2 + SO2 + 2H2O → 2HI + H2SO4 (120°C)
2H2SO4 → 2SO2 + 2H2O + O2 (830°C)
2HI → I2 + H2 (320°C)
As the sulfur and iodine compounds are recovered and reused, the exhaustible material of this cycle is water and the products are hydrogen and oxygen, both of which are fuel for fuel cells. The cycle needs a heat source as high as 830°C.
In 2003, the Japan Atomic Energy Agency conducted successful experiments with the S-I cycle.[6] Their plant produces 35-liter hydrogen per hour. They intend to use nuclear reactors to produce hydrogen. Why do they adhere to nuclear power? The operation temperature of the current nuclear power generators is not high enough to utilize this cycle and the Very-High-Temperature Reactor whose outlet temperature reaches 1000°C is yet to be built. The Very High-Temperature Reactor is not a promising heat provider, because the plant will be so remote from consumers that it will cost high to transport hydrogen to them. Without building new nuclear plants, the high heat source will be near us. A kind of fuel cell operates near 1000°C and its cogeneration can utilize the S-I cycle, minimizing the distance between the producer and the consumer of hydrogen.
Recently researchers at Purdue University have discovered a more innovative method to produce hydrogen. According to their announcement, just adding water to an alloy of aluminum and gallium can produce hydrogen.
When water is added to the alloy, the aluminum splits water by attracting oxygen, liberating hydrogen in the process. The Purdue researchers are developing a method to create particles of the alloy that could be placed in a tank to react with water and produce hydrogen on demand.[7]
You can make both aluminum and gallium from the raw mineral bauxite. At present gallium is scarce, but this catalyst is inert and reusable. The real problem is the deoxidization of alumina. As the alloy reacts with water, the aluminum turns into alumina (aluminum oxide). It costs a lot to recycle alumina back into aluminum. Moreover, the use of carbon electrodes to deoxidize alumina emits carbon dioxide.
2Al2O3 + 3C → 4Al + 3CO2
Whether this technology is competitive with gasoline or not is still uncertain.
3.2. How to Store and Transport Hydrogen
Compared with oil and coal, hydrogen gas has good energy density per weight, but poor energy density per volume. You can use pipelines to feed hydrogen into stationary fuel cells, but you must compress hydrogen in some way to feed it into fuel cells in mobile devices.
One of the ways to compress hydrogen is to cool it down to liquid. Since the boiling point of liquid hydrogen is as low as 20.268K, cooling consumes 20% of hydrogen energy. Even at the low temperature, it boils off 1% a day and the tank storing liquid hydrogen must be well insulated to prevent the boil off. Though this method costs high, liquid hydrogen has by far worse energy density per volume than gasoline.
High pressure can also compress hydrogen, but this method has the following problems. First, compressing a gas requires energy to power the compressor. Second, the high-pressure hydrogen tank is dangerous, when some accidents break the tank. Third, high pressure accelerates hydrogen embrittlement. Hydrogen atoms are so small that they can be trapped in the lattice of the metal, migrate through it, react with impurities and worsen the properties of the material. The higher the pressure becomes, the more severe the hydrogen embrittlement becomes.
In 2004, Mitsubishi Corporation developed HHEG (High-pressure Hydrogen Energy Generator) that compresses hydrogen gas without a compressor through its prototype electrolyzer. The HHEG can solve the first problem but other problems are yet to be solved. Hydrogen embrittlement is especially the most serious problem.
Utilizing metal alloy makes the best of the hydrogen embrittlement. Some alloys, for example, vanadium alloy, absorb hydrogen at high pressure and emit it at low pressure with relatively less damage by hydrogen embrittlement. The conditions of the ideal metal alloy are abundant, inexpensive, and light in addition to absorbing much hydrogen, operating at a lower temperature, and catalyzing the absorption/emission reaction. No such ideal alloy that can be commercialized has ever been found.
The metal alloys for hydrogen storage are interstitial hydrides, while hydrogen can be stored as ionic hydrides or covalent hydrides. Among ionic hydrides, sodium borohydride is the most promising. Sodium borohydride is a sodium tetrahydroborate whose aqueous solution emits hydrogen.
NaBH4 + 2H2O → NaBO2 + 4H2
This reaction needs no precious catalyst. Seiko Instruments Inc. found malic acid catalyzes dehydrogenation more effectively than platinum. It supplies hydrogen quickly at room temperature.
The solution is less flammable and less volatile but much more corrosive than gasoline. Borohydride itself is solid and difficult to handle. Because the waste borax or sodium metaborate (NaBO2) is harmful, it should be hydrogenated back into borohydride, but it requires much energy to recycle.
Sodium borohydride costs high currently, but, as the material of borohydride, boron is also abundant and cheap, recycling and mass production will reduce the price drastically.
A group of covalent hydrides, such as cyclohexane, decalin, and methylcyclohexane, can be easily recycled after dehydrogenation. Masaru Ichikawa named them “organic hydrides".[8]
When benzene, naphthalene, and toluene come in contact with Pt catalysts, they store hydrogen and are chemically transformed into organic hydrides, respectively cyclohexane, decalin, and methylcyclohexane. They are returned to the original aromatic compounds after the hydrogen is extracted and can be reused.
Benzene is toxic. Naphthalene is solid at room temperature and pressure and difficult to handle. Ichikawa regards toluene-methylcyclohexane as the best combination. The toluene and methylcyclohexane are liquid at atmospheric temperature and pressure. Their physical property is similar to that of gasoline (in fact, methylcyclohexane is a component of jet fuel). This means they enable us to use the conventional infrastructure, such as gas stands, tank trailers, cargo trains, shipping tankers, and so on.
The following shows the hydrogenation of toluene.
C7H8 + 3H2 → C7H14 + 215kJ/mol MCH
Currently, 5 kilograms of hydrogen is required for a fuel-cell car to cover a distance of 500 kilometers. This amount is equivalent to 56,000 liters of hydrogen gas at room temperature and pressure, but it can be stored in about 70 liters of an organic hydride. That is to say, the volume of hydrogen gas is reduced to 1/800 to 1/1,000 when it is chemically stored in the organic hydrides. The organic hydrides are the medium of hydrogen storage/supply capacity that has attained the DOE and USCAR target values for FC car application in the U.S.
The demerit of organic hydrides consists in that their hydrogenation and dehydrogenation require a platinum catalyst. As platinum is expensive and scarce, it will hinder organic hydrides from prevailing.
As the dehydrogenation of organic hydrides is an endothermic reaction, heat must be added to organic hydrides. This does not matter if fuel cells operate at a temperature higher than 300°C.
Robert Crabtree of Yale University considers it impractical that organic hydrides require high temperatures to unlock hydrogen. So, he proposes incorporating nitrogen into his organic liquids.
Nitrogen binds to hydrogen less strongly than carbon does, and the presence of nitrogen within a carbon-based ring weakens the remaining C-H bonds. These weakened bonds make it easier to get hydrogen out as the liquid passes over a catalyst, and lower operating temperatures would be needed — the material only needs to be raised by 50 degrees Celsius.[9]
I expect more and more new technologies will appear to improve the storage and transportation of hydrogen.
3.3. How to Generate Electricity
There are many kinds of fuel cells and we can choose the most appropriate one for the specific applications. First, we can divide applications into two, stationary and mobile.
For a stationary application, namely, to use electricity in houses, offices, factories and so on, SOFC (Solid Oxide Fuel Cells) are the most promising.

At the anode’s side, the oxygen ions and the hydrogen produce water and electricity. The air and the electricity flow into the cathode. Oxygen in the air is ionized and goes through the ceramic electrolyte. SOFC are mainly made of ceramic.
SOFC operate at very high temperatures, typically between 700 and 1,000ºC. Thanks to their high operating temperature, they can achieve the highest efficiency of all fuel cells, have no need for expensive catalysts, and can reform internally various fuels such as methane, propane, butane, carbon monoxide without getting damaged. So, you can use the ready-made pipeline infrastructure that feeds SOFC with town gas.
The high temperature of SOFC shortens their lives and requires expensive heat-resistant materials. How to make them durable is the problem to be solved. Another problem is that they take a long time to start up. This is not so problematic when they operate constantly.
TOTO Ltd. developed a microtubular SOFC for a mobile application that operates at 500ºC and starts up in 5 minutes. Is this SOFC appropriate for mobile use? You cannot put on something that operates at 500ºC. As the ceramics are fragile, they are unsuitable for automobile engines and drivers cannot wait for engines to start up in 5 minutes.
For mobile applications, PEFC (Polymer Electrolyte Fuel Cells) is suitable. PEFC operate at low temperatures, typically between 70 and 90ºC. Thanks to this low temperature, the stacks are durable and the startup time is short. On the other hand, they require platinum as a catalyst and pure hydrogen as fuel. Internal reforming is impossible and their energy efficiency is low.
Acta S.p.A., an Italian company, developed new DEFC (Direct Ethanol Fuel Cells), which are based on non-noble metals. They include mixtures of Fe, Co, Ni at the anode, and Ni, Fe, or Co alone at the cathode. Though the fuel is ethanol, their power densities achieved 140 mW/cm2 at 0.5 V at 25°C.
Because ethanol is fed directly into them, complicated catalytic reforming is unnecessary. The storage of ethanol is much easier than that of hydrogen, for it is liquid at room temperature and pressure. Moreover, ethanol is safe enough to drink, since it is the alcohol found in alcoholic beverages.
As ethanol can be blended with gasoline, it has been studied as an alternative fuel for automobiles. The supply of ethanol is limited, however, so long as it is produced from edible food, such as sugarcane and corn. In 2006, Honda and RITE (Research Institute of Innovative Technology for the Earth) developed a new method to produce ethanol from the cellulosic biomass, such as leaves, stalks, and straws of plants, which are currently discarded. Whatever the purpose is, ethanol will be produced in bulk and the fuel for DEFC will get more inexpensive and abundant than today.
DEFC are kind of PEFC but resemble SOFC in that oxygen is ionized and goes through the anion-exchange membranes. The reaction is as follows.
Cathode side: 3O2+6H2O+12e–→12OH–
Anode side: C2H5OH+12OH-→2CO2+9H2O+12e–
The released carbon dioxide is offset by the carbon dioxide captured by plants through photosynthesis. So, DEFC are carbon-neutral.
In 2006, Acta S.p.A. demonstrated the durability of DEFC catalyst and the result is as follows.[11]
- A direct ethanol fuel cell for over 1,500 hours at 5mW/cm2 at room temperature
- A direct ethanol fuel cell for over 500 hours at 20mW/cm2 power output at room temperature
- A direct ethylene glycol fuel cell for over 500 hours at 20mW/cm2 at room temperature
The durability is good, but the power density is low. DEFC can be used only for small mobile electronics.
When it comes to high power density fuel cells for mobile applications, DBFC (Direct Borohydride Fuel Cells) are the strongest. They are some sorts of alkaline fuel cells whose fuel is a solution of sodium borohydride.
Here is a diagram of alkaline fuel cells in general.

Unlike classical alkaline fuel cells, DBFC are not poisoned by carbon dioxide in the air. They decompose and oxidize the borohydride directly, sidestepping hydrogen production. The reaction is as follows.
Cathode: 2O2 + 4H2O + 8e– → 8OH–
Anode: NaBH4 + 8OH– → NaBO2 + 6H2O + 8e–
The power density of DBFC that employ hydrogen peroxide as oxidant is about 350 mW/cm2 at the cell voltage of almost 1.2 V at 70°C.[13] DBFC do not need expensive catalysts and their fuel can be inexpensive enough to be commercialized.
I have already pointed out the demerits of borohydride. The reaction of DBFC emits no carbon dioxide but produces waste borax (NaBO2), which requires much electricity to be hydrogenated back into borohydride. DBFC are suitable for larger mobile electronics, but not for automobiles, because borohydride is not liquid.
For automobile applications, organic hydrides are promising. RDHFC (Rechargeable Direct Hydride Fuel Cells), a kind of PEFC directly fed with organic hydrides, were developed for the mobile application. The power density of RDHFC is, however, as low as that of DEFC. DBFC produce higher energy yield than their indirect ones, while RDHFC produce lower energy yield. Since organic hydrides are easy to be recycled, RDHFC can be recharged, as the name reads.
RDHFC is expensive because both hydrogenation/dehydrogenation of organic hydrides and the reaction of PEFC need platinum as a catalyst.
3.4. How to Commercialize Fuel Cells
Mass production of fuel cells will reduce the price of fuel cells if they do not include platinum and other precious materials. Fuel cells do not sell currently, because they are too expensive, and manufacturers cannot produce them on a large scale, because they do not sell. How can we escape from this vicious circle?
The current fuel cells cannot beat internal combustion engines but can compete with batteries. Fuel cells are powerful and have a long life, compared with batteries. It takes quite a long time to charge a battery for a mobile computer or a mobile phone. Mobile devices use direct currents to make themselves small. The grid adopts alternating current and converting alternating current into direct current loses some energy. Charging fuel cells with fuel takes little time and there is no energy loss for conversion because fuel cells generate direct current. Fuel cells will find a breakthrough into the market in the mobile application.
The next target of commercialization is the stationary application. You can charge SOFC with natural gas through ready-made pipelines. SOFC fits houses or factories that demand a lot of heat. On the other hand, the fuel of PEFC is pure hydrogen and you cannot use most of the ready-made pipelines to transfer hydrogen because of the hydrogen embrittlement. Plating the inside of the pipelines with special alloy can prevent hydrogen embrittlement, but it takes time and cost a lot.
It takes a longer time to apply fuel cells to automobiles. Some assume that a chain of hydrogen-equipped filling stations and other infrastructure along a road or highway that allow hydrogen-powered cars to travel is necessary for the mass utilization of hydrogen cars. The fastest way to spread hydrogen cars is to adopt organic hydrides, especially, methylcyclohexane, as carrying media. Because the physical property of methylcyclohexane is very similar to gasoline, you can utilize the existing infrastructure such as filling stations, tank-lorry, cargo-train, shipping tanker, and so on. Organic hydrides are useful for stationary applications when consumers are located in so rural areas that pipelines are unavailable.
4. Other Candidates
Thermal power generation is the most inexpensive and commonly used. But the fuel for it, namely oil, coal, and natural gas are limited resources and destined to be exhausted someday if we do not reduce consumption drastically. Moreover, the combustion of these causes various kinds of pollution.
Though oil is the main fuel for today’s thermal power generators, we must continue to use it as the material for plastics, fibers, and other chemical products. So, we should seek another clean power generation that does not waste such useful limited resources.
Besides fuel cells, there are many candidates for it, such as nuclear power, photovoltaic power, wind power, water power generators. Let’s examine them to see whether these are superior to fuel cells or not.
4.1. Nuclear Power Generation
Nuclear power generates electricity by using heat released from nuclear fission to boil water, produce steam, and drive a steam turbine. Though the use of nuclear power is still controversial, today it is re-evaluated, because it is believed to emit no greenhouse gas. For example, Patrick Moore, co-founder of Greenpeace, who once opposed nuclear power, now supports it.
More than 600 coal-fired electric plants in the United States produce 36 percent of U.S. emissions ― or nearly 10 percent of global emissions ― of CO2, the primary greenhouse gas responsible for climate change. Nuclear energy is the only large-scale, cost-effective energy source that can reduce these emissions while continuing to satisfy a growing demand for power. And these days it can do so safely.[14]
Actually, nuclear power generation indirectly emits carbon dioxide. First, lots of oil must be burned to construct the plants, refine uranium fuel, and so on. Second, nuclear power generation discharges two-thirds of the produced energy as waste heat, namely it throws hot water into rivers or the sea, thus heightening the temperature of the water. The warmer the water becomes, the more carbon dioxide spouts out from it.
Of course, all power plants emit thermal pollution. But unlike fuel cells and gas turbines, nuclear plants are centralized generators far from the heat-consuming area. It is very difficult and restricted to realize the cogeneration of nuclear energy.
Nuclear power produces high-level radioactive waste. It can remain dangerous for thousands of years. The risk of accidents is also high. An example of the worst possible situation is what happened to the Chernobyl Nuclear Power Plant.
How about using nuclear power in space, then? In space, you do not have to care for the environment. Nuclear power is not renewable energy and its resource, uranium-235, is limited. So, it would be wise to reserve these limited resources for future space development instead of consuming them today.
4.2. Photovoltaic Power Generation
Photovoltaic power generation cannot be the main energy resource. It works only on fine days, and it cannot fully operate even in the daytime because, if it hardly rains, dust will pile up on the panel, and generation efficiency will soon drop. Because of this inefficiency, it takes about ten years for photovoltaic power generation to redeem all the energy required to make the set of photovoltaic apparatus. It takes furthermore to redeem the energy to dispose of the set.
Considering that the combustion of oil necessary to make and dispose of the set pollutes the environment, it is hard to say photovoltaic power generation is clean energy. As the performance of photovoltaic apparatus exposed to wind and rain deteriorates gradually, it may get useless or broken when it redeems all the energy debt. That is to say, photovoltaic power generation might turn out not a producer but a consumer of energy.
It is more difficult for the economic income to cover the expenses of photovoltaic power generation than for the energy income. Japanese government bears a third of expenses and finances the rest at low interest, which is, however, higher than the earning rate of electricity generation. It is deplorable that photovoltaic apparatus, which is neither friendly to the environment nor economically reasonable, should sell well promoted by public subsidy and the so-called ecological movement.
4.3. Wind Power Generation
Wind power generators convert wind energy into electricity, using wind turbines. Because wind power generation as well as photovoltaic is at the mercy of variable nature, it needs backup generators and adjusting equipment, which raises the amount of initial investment.
Like nuclear power generation, it cannot adjust electricity output to demand. Demand for electricity culminates in summer when wind power is usually weak. It blows in the nighttime as well as in the daytime, although demand for electricity drops at night. Other power generators must fill the gap between demand and supply.
Since generators will break if the wind gains in force, the wind power must be constant, but there are few areas that fulfill this condition. Appropriate places, if any, are usually far from the electricity-consuming area. In that case, long transmission lines are necessary, which lowers energy efficiency and heightens economic costs. The unit price of wind power generation is not as expensive as that of photovoltaic power generation, still higher than the standard unit price.
You might think that a wind power generator, once set up, generates electricity almost forever, therefore turns out to be efficient in the long run. But actually, the maintenance expense is required and its performance deteriorates gradually owing to friction between an axis and axis bearing, so it is doubtful whether the income meets the expenses.
4.4. Hydroelectric Power Generation
Most hydroelectric power comes from the potential energy of dammed water driving a water turbine and generator. The cost of electricity is high, but other purposes of damming make up for the high cost. The other purposes of dams are providing water (for drinking, irrigation, or industrial use), flood control, improving navigation, creating recreation areas or habitats for fish and wildlife, and so on.
This generation utilizes solar energy evaporating water. Though constructing hydroelectric plants costs high, they generate electricity without fuels and personnel for a long time, once they are completed. Hydroelectric plants have longer lives than fuel-fired generators. Some plants built 50 to 100 years ago are still in service.
However, hydroelectric power generation has several bad impacts on the environment. A dam interrupts the sediment flow of a river, holds the sediment at the bottom of the reservoir, and releases surface water containing very little sediment down the river. This interruption impoverishes downstream soil and promotes soil erosion. Aswan High Dam built in 1970 succeeded in controlling annual floods but has hindered the deposit of nutrient-rich silts on alluvial soils. Today, Egyptian farmers have to apply fertilizer and the Nile Delta is shrinking for lack of silt deposits.
The interruption also impoverishes the sea. Further, a dam also makes it difficult for species, such as salmon and eels to migrate to spawn at their destination. Damming up the flow of the river cuts off the circulation of nutrition that flows from mountains via rivers to seas to enrich fishery resources, which are in turn eaten by animals, which reduce their excrement and dead bodies as nutrition to the mountains. So, dams destroy not only sunken areas but also extended areas including mountains and seas.
I have examined four major candidates for power generators. Each has its own merits and demerits. In my opinion, the most desirable alternative energy resources and power generators are biomass and fuel cells.
5. References
- Jeremy Rifkin. The Hydrogen Economy.
- Ryan O’Hayre, Suk-Won Cha, Whitney Colella, Fritz B. Prinz. Fuel Cell Fundamentals.
- Yun Wang, Ken S. Chen. PEM Fuel Cells: Thermal and Water Management Fundamentals.
- ↑U.S. Department of Energy (2007) Types of Fuel Cells, Hydrogen, Fuel Cells and Infrastructure Technologies Program.
- ↑Michael Kanellos (2007) Hydrogen fuel cells power Fujitsu data center, CNET News.com.
- ↑Fuel Cells 2000 (2007) Fuel Cell Basics: Benefit.
- ↑Jeremy Rifkin (2002) The Hydrogen Economy: The Creation of the Worldwide Energy Web and the Redistribution of Power on Earth, Jeremy P. Tarcher. p.9
- ↑Ma, Hongtao, Ryoichi Kojima, Satoshi Kikuchi, and Masaru Ichikawa. “Effective Coke Removal in Methane to Benzene (MTB) Reaction on Mo/HZSM-5 Catalyst by H2 and H2O Co-Addition to Methane." Catalysis Letters 104, no. 1–2 (October 1, 2005): 63–66.
- ↑Japan Atomic Energy Research Institute. “Successful Demonstration of Hydrogen Production by Thermal Cracking of Water―Thermochemical Water-Cracking Method Featuring No Electrolysis―." August 21, 2003.
- ↑Purdue University (2007) “Engineers perfecting hydrogen-generating technology." August 27, 2007.
- ↑Yasushi GOTO, Tadashi UTAGAWA, Masaru SAKURAMOTO, Yasunori SUGAI, Nobuko KARIYA, Atsushi FUKUOKA, Masaru ICHIKAWA. “5-3. Research and Development of Hydrogen storage and supply system using Organic Hydrides (2): Demonstration studies of hydrogen supply reactor connected to fuel cell." Proceedings of the Annual Conference of The Japan Institute of Energy. August 01, 2002 – August 02, 2002.
- ↑Katharine Sanderson (2007) Hydrogen fuel goes liquid – Nitrogen unlocks the possibility of convenient clean fuels, [email protected].
- ↑U.S. Department of Energy (2007) U.S. Department of Energy (2007) Types of Fuel Cells, Hydrogen, Fuel Cells and Infrastructure Technologies Program.
- ↑Acta S.p.A. (2006) AGM Statement – Acta achieves technical targets.
- ↑U.S. Department of Energy (2007) Types of Fuel Cells, Hydrogen, Fuel Cells and Infrastructure Technologies Program.
- ↑Ramanujam K. Raman et al (2004) A High Output Voltage Direct Borohydride Fuel Cell, Electrochemical and Solid-State Letters, Volume 7, Issue 12, pp. A488-A491.
- ↑Patrick Moore (2006) Going Nuclear – A Green Makes the Case, The Washington Post, April 16, Page B01.








Discussion
New Comments
No comments yet. Be the first one!